group 3: Integrative biology of neuroregeneration
About
We investigate molecular mechanisms that underlie neurodegeneration and neuroregeneration processes in the context of spinal cord pathologies, in particular spinal cord injury (SCI). SCI has currently no therapy leading to even partial recovery. The absence of functional recovery due to the lack of spontaneous axonal regeneration is attributed, among other factors, to the formation of a glial scar, mainly composed of astrocytes and microglia that forms both physical and chemical barriers. However, glial cells concomitantly play beneficial roles on axonal regrowth.
We are developing a multimodal approach with the final aim of enhancing axonal regeneration following spinal cord injury.
Our first research axe is to develop an integrative cell-specific genomic analysis to identify genes responsible of the dual role of glial cell following SCI. Once identified, we modulate gene expression specifically in glial cells in order to enhance their beneficial roles and reduce their negative impacts on axonal regeneration.
The second research axe in the laboratory is to develop translational tools. Concomitantly to behavioral analysis of motor activity, we are developing the use of Magnetic Resonance Imaging (MRI) to follow spinal cord injured animals. Indeed, MRI is a non-invasive method that provides relevant longitudinal assessment of anatomical and structural alterations induced by an injury and the only method used to assess the impact of SCI in human. This project is developed together with the recent BioNanoNMRI platform at the University of Montpellier that has a 9.4T MRI apparatus designed for small animals.
Finally, we apply histological analysis to possibly correlate molecular, cellular, and tissues alterations with functional recovery.
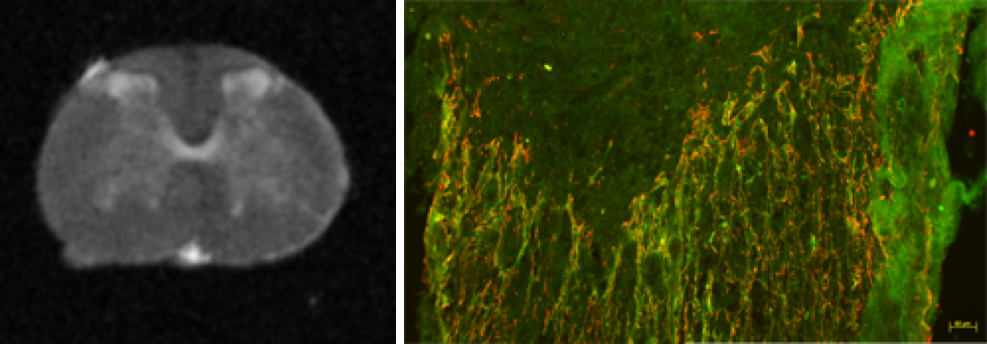
Legends
Left: Ex vivo MRI image of a non-injured spinal cord.
Right: Glial scar forms following spinal cord lesion. In green, astrocytes and in red microglia.
Group
Florence Perrin, scientific officer
 |
Professor University of Montpellier Membre de l'Institut Universitaire de France Contact: florence.perrin(at)umontpellier.fr |
Nicolas Lonjon
 |
PUPH Contact: n-lonjon(at)chu-montpellier.fr |
Gaétan Poulen
 |
Clinicien Attaché, PHU Contact: g-poulen(at)chu-montpellier.fr |
Yannick Gerber
 |
Post-doctorant, Université de Montpellier Contact : yannick.gerber(at)ibn-lab.com |
Chloé Gazard
 |
PhD student Contact : chloe.gazard(at)umontpellier.fr |
References
- Gerber YN, Sabourin JC, Rabano M, Vivanco M, Perrin FE (2012). Early functional deficit and microglial disturbances in a mouse model of amyotrophic lateral sclerosis. PloS One. 7(4): e36000.
- Gerber YN, Privat A, Perrin FE (2013). Gacyclidine improves the survival and reduces motor deficits in a mouse model of amyotrophic lateral sclerosis. Frontiers in Cellular Neurosciences. (7):280.
- Desclaux M, Perrin F, Do-Thi A, Prieto-Cappellini M, Gimenez y Ribotta M, Mallet J, Privat A (2015). Lentiviral-mediated silencing of GFAP and vimentin in reactive astrocytes improves functional recovery and axonal plasticity after spinal cord injury. Journal of Neuroscience Research. 93(1):43-55.
- Noristani* HN, Lonjon* N, Cardoso M, Le Corre M, Chan Seng E, Captier G, Privat A, Coillot C, Goze-Bac$ C, Perrin$ FE (2015). Correlation of in vivo and ex vivo 1H-MRI with histology in two severities of mouse spinal cord injury. Frontiers in Neuroanatomy, doi: 10.3389/fnana.2015.00024.
- Noristani* HN, Sabourin* JC, Gerber YN, Teigell M, Sommacal A, Vivanco M, Webber M, Perrin FE (2015). Brca1 is expressed in human microglia and is dysregulated in human and animal model of ALS. Molecular Neurodegeneration, 10:34, doi:10.1186/s13024-015-0023-x.